MS-PS1-1 focuses on Matter and its Interactions, specifically emphasizing on atomic composition models. In this post, we’ll look at the standard and understand its performance expectation, and we’ll also look at how to make sense of it with students by connecting the dots and scaffolding up with different tasks.
Performance Expectation
Each standard contains a performance expectation that details how students can perform to show what they’ve learned. It describes what students can do, as opposed to what they understand.
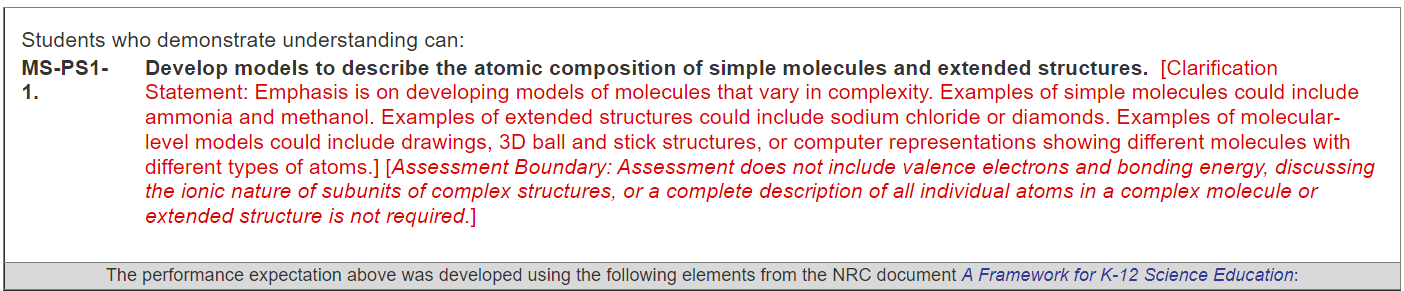
So, with MS-PS1-1, the first thing we need to consider is that this standard asks students to develop models to describe the atomic composition of simple molecules and extended structures. The clarification statement talks about how models can vary in complexity. For example, ammonia and methanol.
Extended structures could be sodium chloride or diamonds, which are very, very simple, basic structures.
The Three-Dimensions
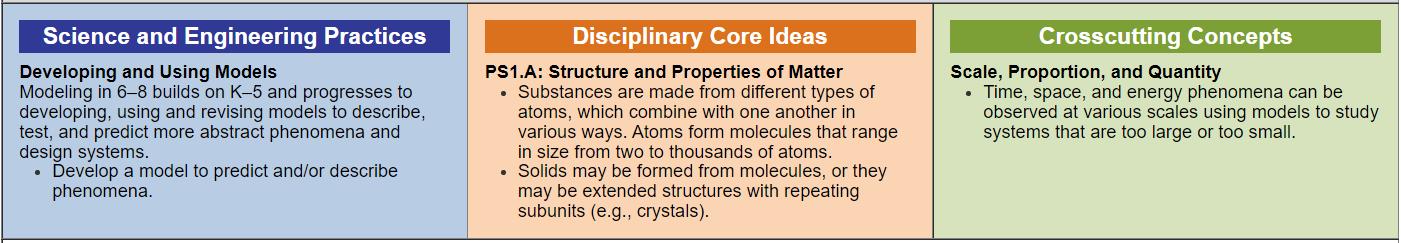
- The SEP for this performance expectation is developing and using models, and more specifically, students are asked to create a model to predict or describe phenomena.
- There are two elements of the DCIs. Students must explain that substances are made of different atoms that combine differently and that molecules can form solids, or they may be extended structures with repeating subunits. For example, diamonds and extending structures of carbon versus water, or ice, which can be H2Os locked together in a solid,
- Finally, the Cross-Cutting Concept in this PE is scale, proportion, and quantity. Students must make connections across seemingly disparate disciplines or situations through scale, proportion, and quantity.
The Concepts
By the end of the unit, students should achieve the following milestones:
-
- Understand that to see macroscopic phenomena (things you can see with your own eye), there are things at smaller scales, at the atomic scale, and the molecular scale, where we can tell what’s going on.
- Visually depicting how atoms come together to make molecules.
- Explain and model that extending structures can form solids as molecules or atoms that are locked together.
- Finally, they should be able to talk about how things can be seen as solid at the macroscopic scale and understand how solids are composed if we get down to a microscopic scale or a molecular and atomic scale. And then comprehend visible reactions by going down to the atomic-molecular level to describe those phenomena.
There are different ways to assess students on MS-PS1-1:
- Ask them to model a molecule you’ve already discussed in class. A good formative assessment might be: “Draw a model of a water molecule that has two hydrogens and one oxygen” or “Draw a model of a carbon dioxide molecule that has two carbons or one carbon and two oxygens.”
- Explain how atoms can form diamonds, graphite, or carbon-only structures. Crystalline structures or structures where a single atom or molecule is repeated would be an excellent example. An assessment question might be: Draw a dry malt model of those repeating subunits.
- If you want students to make sense of phenomena, we’ll ask them to model something they have never seen before. You can briefly define some of the characteristics or properties of the phenomenon, but we won’t tell them what the model looks like. Students have to develop a model that they have never seen before to explain how simple molecules or extended structures are just different combinations of atoms.
Student Activity
Once students understand the concepts, they can develop models to describe the atomic composition of simple molecules and extended structures. For example, they could create a model of water made up of hydrogen and oxygen atoms. Or they could create a model of DNA made up of nucleotide molecules.
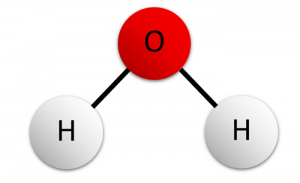
We could build these molecular-level models with drawings or 3D balls and sticks or computer representations. We want students to show atoms and the atoms linked together to form molecules. The assessment boundary on the standard says that students should not include valence electrons bonding energy, no ionic subunits, and they should not describe all individual atoms in a complex molecule.
The key here is that students need to understand the concept of atoms, molecules, and extended structures before developing models to describe them. Once they have that foundation, they can build more complex models.
So students may also build models of things like water (H2O), ammonia (NH3), carbon dioxide (CO2), or methane (CH). These molecules are simple structures, allowing them to show how different atoms fit together to form a molecule.

Assessment
There are different ways to assess students on MS-PS1-1:
- Ask them to model a molecule you’ve already discussed in class. A good formative assessment might be: “Draw a model of a water molecule that has two hydrogens and one oxygen” or “Draw a model of a carbon dioxide molecule that has two carbons or one carbon and two oxygens.”
- Explain how atoms can form diamonds, graphite, or carbon-only structures. Crystalline structures or structures where a single atom or molecule is repeated would be an excellent example. An assessment question might be: Draw a dry malt model of those repeating subunits.
- If you want students to make sense of phenomena, we’ll ask them to model something they have never seen before. You can briefly define some of the characteristics or properties of the phenomenon, but we won’t tell them what the model looks like. Students have to develop a model that they have never seen before to explain how simple molecules or extended structures are just different combinations of atoms.
Summary
The key is for students to understand that everything is made up of atoms and molecules, even things we can see with our own eyes. Once they have that foundation, they can develop models to explain the world around us at different scales. And with those models, they’ll be making sense of the phenomena they see daily.
We hope this post helps you plan how to instruct and assess these specific topics. If you’d like to try InnerOrbit with your students, sign up for a free trial to build assessments from over 9,000 phenomena-driven questions, meticulously tagged, to deliver the most detailed data possible on SEPs, DCIs, and CCCs.


