HS-PS1-1 focuses on Structure and Properties of Matter, specifically emphasizing that models of the outer electron shell can be helpful in explaining the relative properties of elements. In this post, we’ll break apart the standard and to better understand the performance expectation, and we’ll also look at how to make sense of this topic with students by scaffolding up with different phenomena and tasks.
Performance Expectation
Each standard contains a performance expectation that details how students can perform to show what they’ve learned. It describes what students can do, as opposed to what they understand.

With HS-PS1-1, the first thing we need to consider is that this standard asks students to “Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms.” The clarification statement expands that these properties might be reactivity or metals, types of bonds formed, number of bonds, and oxidation reactions. For example, Helium has a full valence electron shell and therefore has very low reactivity.
We can contrast Helium to Sodium, which is a metal with a single valence electron. This makes sodium highly reactive and enables the element to easily form both ionic and metallic bonds.
The Three-Dimensions

- The SEP for this performance expectation is developing and using models, and more specifically, students are asked to use a model to predict or describe the relationships between systems or between components of a system.
- There are two elements of the DCIs. Students must understand that atoms are made of a charged nucleus that’s made of protons and neutrons, and this nucleus is surrounded by electrons. Students also need to understand that the periodic table organizes elements horizontally and vertically based on the relative properties of elements.
- Finally, the Cross-Cutting Concept in this PE is Patterns. Students must make connections between the patterns that can be observed at different scales to provide evidence for causality. In this context, we’re specifically using the atomic level properties of elements to better explain the bulk behavior and bonding of those elements.
The Goals
By the end of a lesson on this standard, students should achieve the following milestones:
-
- Understand subatomic structures and their relative properties: Electrons, Neutrons, Protons.
- Understand atomic models and how they show theses subatomic structures.
- Understand the role of valence electrons in the properties of atoms and how the periodic table organizes elements.
- Finally, they should be able to use atomic models and elements’ placement on the periodic table to be able to explain the properties of elements and/or their placement on the periodic table.
There are many ways to give students phenomena to better understand and apply their understanding around HS-PS1-1. Here are 3 example phenomena you can use:
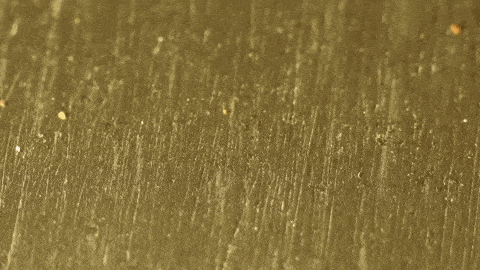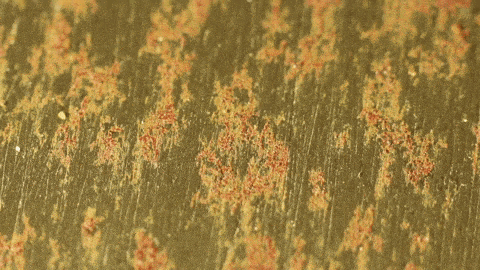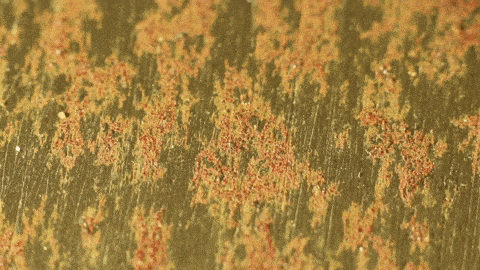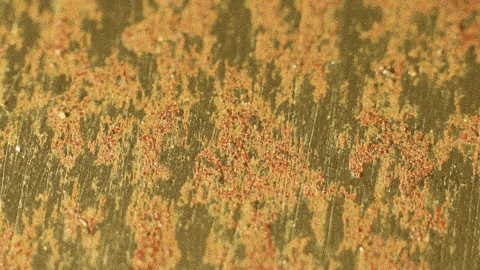
- Ask students to explain rust formation on Iron, through the lens of valence electrons. You can give students a Bohr model of iron atoms and ask them why iron oxidizes so much more easily than other elements. What types of bonds form in this reaction? Why?
- Explain how Neon atoms emit light, but are non-reactive. An assessment question might be: Draw a model of Neon and Oxygen atoms. Use your model to explain the reactivity of both elements, through the lens of their valence electrons.
- Sodium Chloride formation! Ask students to draw or analyze models of these two elements to explain why they form such salty ionic bonds. How is this behavior different from the formation of Covalent bonds?
If you want students to make sense of phenomena, it’s crucial to ask them to look at multiple different phenomena and use questions or phenomena like the ones above to give students ways to build their understand of NGSS phenomena and apply that understanding ti make sense of new phenomena. This means: ask them to model or analyze something they have never seen before!
Student Assessment for HS-PS1-1
Once students understand the concepts from HS-PS1-1, they can develop or analyze models to explain the properties of those elements, through the lens of valence electrons. For example, they could analyze 4 different elements macroscopic behavior and oxidation to explain how the valence electrons of these 4 elements either make them more or less likely to oxidize/rust.

You could give students the following elements:
1. Copper
2. Gold
3. Iron
4. Aluminum
Ask students to create Bohr models of each elements and use evidence from their models to explain why each elements will or will not oxidize (or rust). Compare each element with a Bohr model of Oxygen to explain the types of bonds that can be formed.
This could be a hands on lab, where students construct 3-dimensional models or you could give them paper to draw their models on. Scaffold as needed for your students by giving pre-constructed models or helping them to model the analysis of on of these elements.
Summary
The key is for students to understand that everything is made up of atoms and those atoms are organized into the periodic table. Once they have that foundation, they can develop models to explain the relationship between valence electrons and properties of elements. Applying those models beyond the classroom will give students the curiosity and tools to make sense of the physical science phenomena they see daily.
We hope this post helps you plan how to instruct and assess these specific topics. If you’d like to try InnerOrbit with your students, sign up for a free trial to build assessments from over 10,000 phenomena-driven questions, meticulously tagged, to deliver the most detailed data possible on SEPs, DCIs, and CCCs.