HS-PS1-2 focuses on Structure and Properties of Matter and Chemical Reactions, specifically emphasizing explaining the outcomes of chemical reactions of main group elements. In this post, we’ll look at the standard to clarify and break down the performance expectation, and we’ll also look at how to make sense of it with students by scaffolding up with different tasks in instruction and assessment.
Performance Expectation
Each standard contains a performance expectation that details how students can show what they’ve learned. It describes what students can do, as opposed to what they understand.
![HS-PS1-2. Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties. [Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical reactions involving main group elements and combustion reactions.]](https://blog.innerorbit.com/app/uploads/2024/07/Screenshot-2024-07-24-at-10.58.05 AM.png)
In HS-PS1-2, the first thing we need to consider is that this standard asks students to construct an revise an explanation. In order for us to meet the standards, students will need opportunities to build en explanation and then revise their explanations. All of their explanations should center around chemical reactions and how the periodic table, valence electrons, and patterns in chemical properties impact those reactions.
The clarification statement helps to refine the PE further, by describing some simple reactions that students should aim to explain. The Assessment boundary also narrows our scope to focus on main group elements and combustion reactions.
The Three-Dimensions

- The SEP for this performance expectation is Constructing Explanations and Designing Solutions, and more specifically, students are asked to construct and revise an explanation, using evidence from a variety of sources that show how scientific theories and laws can be use to explain phenomena.
- There are two elements of the DCIs. Students must explain that the periodic table orders elements by properties and valence electrons and that we can use the chemical properties of elements to predict how they will react. For example, there are similarities in the valence electrons in both Sodium (Na) Potassium (K), so we can expect both to form ionic compounds with Chlorine.
- Finally, the Cross-Cutting Concept in this PE is Patterns. Students must make connections across different levels of scale (i.e. the atomic scale and the macroscopic scales) to provide a causal explanation for phenomena. An example of this is the patterns in properties we see, given atoms with the same outer electron shell.
The Goals
By the end of a unit or lesson covering this standard, students should achieve the following milestones:
- Understand that the periodic table organizes atomic properties.
- Atomic properties can be used to predict outcomes of chemical reactions.
- Construct an explanation to predict outcomes of reactions, given different atoms.
- Finally, they should be able to revise their explanations, given evidence and scientific laws.
There are different ways to assess students on HS-PS1-2:

- An accessible starting reaction might be combustion of Methane, trapped under ice in a frozen lake, as students are likely familiar with combustion reactions in their life experience. We can give students models of the reactants of methane combustion and ask them to explain what products might be able to form from those elements.
- We could also ask students to predict what will happen in the electrolysis of water, when we produce hydrogen fuel. By pushing a current through the water, electrons are introduced that can break the bonds between Hydrogen and Oxygen in water and allow those atoms to form into different compounds: H2 and O2. An assessment question might be: Explain why the electrolysis of water allows us to create hydrogen gas.
- We can then return to the original combustion reaction by asking: What properties does hydrogen gas have that make is useful as a fuel? This allows students to connect their learning back to the initial combustion reaction we used in our first phenomena.
Student Activity
Once students understand the concepts in this performance expectation, we’re ready to give them a phenomena-driven assessment to see if they can apply their knowledge and skills to make sense of a novel phenomenon. For example, we could ask students to explain neutralization through the lens of drain cleaners. Most things that get stuck in drains are acidic, so how would sodium chloride help to neutralize HCl?

You may choose to provide students with the reactants and products, as you see below, or you may choose to remove that scaffold and ask them to determine what would be formed in this neutralization reaction.
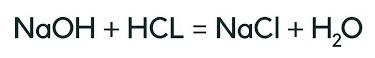 The key here is that students need to understandhow the structure of each atom will impact their ability to neutralize the reactants and form Sodium Chloride salt (NaCL) and water (H2O) molecules.
The key here is that students need to understandhow the structure of each atom will impact their ability to neutralize the reactants and form Sodium Chloride salt (NaCL) and water (H2O) molecules.
You may choose to add in a hand on task or allowing students to build physical models of the reactants and product, but in the end, they should be able to explain how their understandings of atomic structure can be used to make sense of this reaction.
Summary
The key is for students to understand that the periodic table organizes elements and that the properties of those elements (i.e. their valence electrons) determine their properties and the bonds they form. Once students have that foundation, they build an explanation of how those properties are applied in novel reactions.
We hope this post helps you plan how to instruct and assess these specific topics. If you’d like to try InnerOrbit with your students, sign up for a free trial to build assessments from over 10,000 phenomena-driven questions, meticulously tagged, to deliver the most detailed data possible on SEPs, DCIs, and CCCs.


